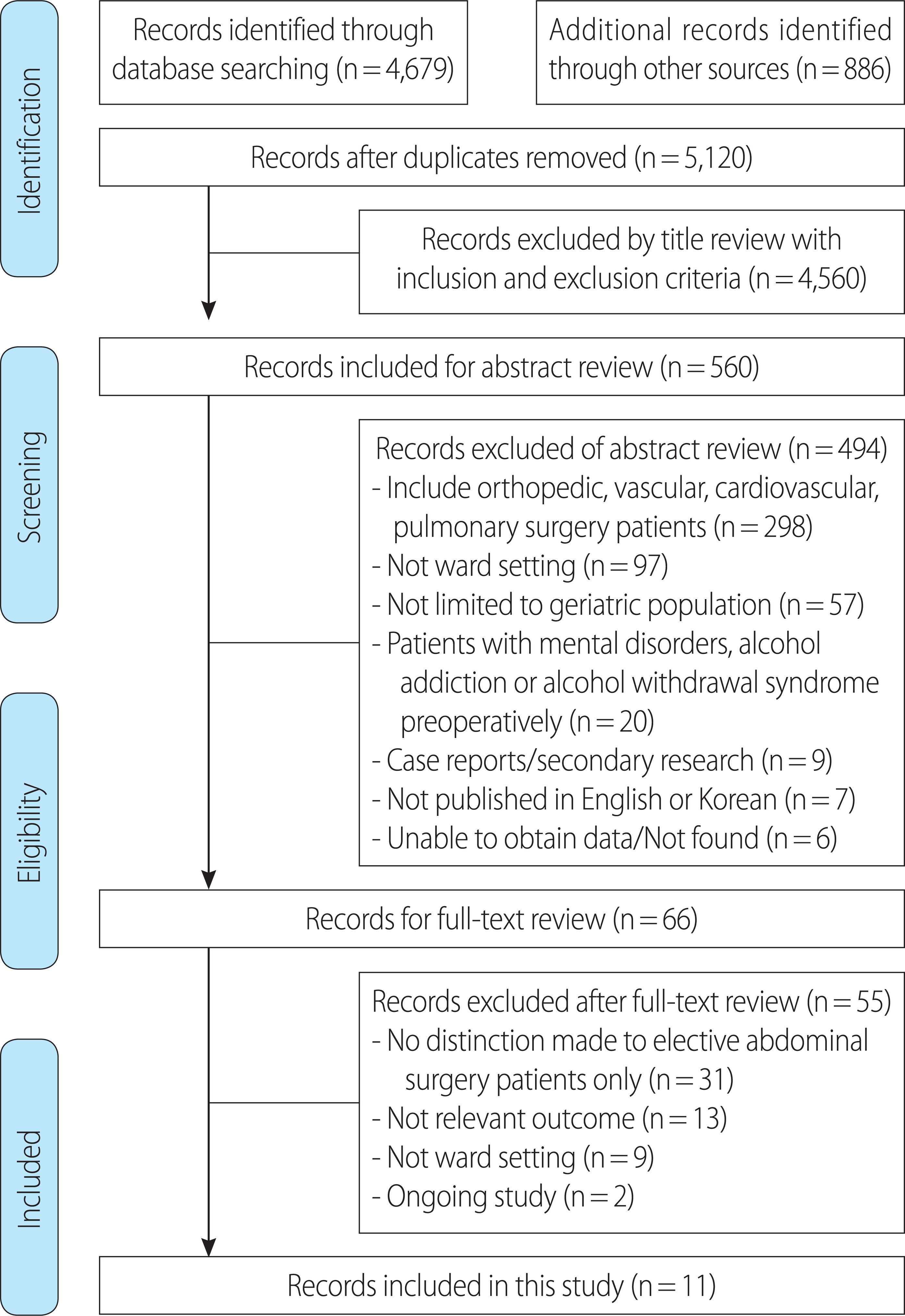
INTRODUCTION
METHODS
1. Search strategy
2. Eligibility criteria
3. Quality appraisal
Table 1.
| No. | Reference | Specific criteria | Overall assessment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Sampling | Sample size justification | Attrition | Measurement | Threat to validity | Statistical analysis | Discussion | Methodological rigor | Data relevance | ||
|
|
||||||||||
| 1 | Olin et al. (2005) | + | - | + | + | - | + | + | H | H |
| 2 | Morimoto et al. (2009) | + | - | - | + | - | + | + | H | H |
| 3 | Koebrugge et al. (2009) | + | - | + | + | - | + | + | H | H |
| 4 | Brouquet et al. (2010) | + | - | - | + | - | + | + | H | H |
| 5 | Patti et al. (2011) | + | - | + | - | - | + | + | H | H |
| 6 | Jia et al. (2014) | + | - | - | - | - | + | + | L | L |
| 7 | Tei et al. (2016) | + | - | - | + | - | + | + | H | H |
| 8 | Ito et al. (2016) | - | - | - | + | - | + | + | L | H |
| 9 | Maekawa et al. (2015) | + | - | + | + | - | - | + | H | H |
| 10 | Xiang et al. (2017) | + | - | - | + | - | + | + | H | H |
| 11 | Chen et al. (2017) | + | - | + | + | + | + | + | H | L |
RESULTS
Table 2.
| No. | Reference | Country | Study design | Sample size (age group) | Surgery type | Study aim | Major findings |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 1 | Olin et al. (2005) | Sweden | Prospective cohort | 51 (65 and over) |
Colorectal Pancreatic Hepatic |
To investigate and identify the occurrence and related factors of postoperative delirium in elderly patients undergoing major abdominal surgery |
Approximately half of the elderly patients in the study developed postoperative delirium. Bleeding was found to be an important risk factor for delirium |
| 2 | Morimoto et al. (2009) | Japan | Prospective cohort | 20 (65 and over) | Colorectal | To evaluate the relationship between patient factors, including cerebral oxygen saturation, and the incidence of postoperative delirium | Patients’ age, low preoperative kana-hiroi test score, and low preoperative cerebral oxygen saturation were important risk factors for postoperative delirium. |
| 3 | Koebrugge et al. (2009) | Netherlands | Prospective cohort | 71 (65 and over) |
Gastric Colorectal Pancreatic Hepatic |
To determine the incidence, risk factors and outcomes of postoperative delirium among patients aged 65 and above undergoing elective abdominal surgery at a surgical ward with a high standard delirium care | At a surgical ward with high standard delirium care, the incidence of delirium was 24%. Age was a significant risk factor for postoperative delirium and mortality was higher in delirious patients undergoing elective abdominal surgery. |
| 4 | Brouquet et al. (2010) | France | Prospective cohort | 83 (75 and over) |
Gastric Colorectal Pancreatic Hepatic |
To determine incidence, duration, and risk factors for postoperative delirium in elderly patients undergoing major abdominal surgery |
Postoperative delirium is a frequent and severe postoperative event in elderly patients after major abdominal surgery. A perioperative geriatric assessment should be recommended to patients with an American Society of Anesthesiologists status of 3-4 and preoperative impaired mobility to facilitate the management of postoperative delirium. In high-risk patients, the postoperative administration of tramadol should be avoided. |
| 5 | Patti et al. (2011) | Italy | Prospective cohort | 100 (65 and over) | Colorectal | To investigate the incidence and risk factors for postoperative delirium in 100 consecutive patients over 65 years who underwent colorectal surgery for carcinoma | Postoperative delirium is a frequent complication after colorectal surgery for carcinoma. |
| 6 | Jia et al. (2014) | China | Randomized clinical trial | 233 (70-88) | Colorectal | To investigate the role of fast-track surgery in preventing the development of postoperative delirium and other complications in elderly patients with colorectal carcinoma |
Fast-track surgery decreases the length of stay, facilitates the recovery of bowel movement, and reduces occurrence of postoperative delirium and other complications in elderly patients with colorectal carcinoma compared to traditional perioperative management. The lower incidence of delirium is at least partly attributable to the reduced systemic inflammatory response mediated by IL-6. |
| 7 | Tei et al. (2016) | Japan | Retrospective cohort | 311 (75 and over) | Colorectal | To determine the incidence of and risk factors for postoperative delirium in patients with colorectal cancer who had undergone laparoscopic colorectal resection | The risk of postoperative delirium is associated with older age, operative approach, organ space surgical site infection. |
| 8 | Ito et al. (2016) | Japan | Retrospective cohort | 146 (70 and over) | Pancreatic | To investigate the incidence and risk factors for delirium after pancreaticoduodenectomy | The Charlson Age Comorbidity Index, especially in elderly patients, was associated with the incidence of postoperative delirium. |
| 9 | Maekawa et al. (2015) | Japan | Prospective cohort | 517 (75 and over) |
Gastric Colorectal Pancreatic Biliary tract Hepatic |
To determine whether carrying out the Comprehensive Geriatric Assessment (CGA) before operations would be useful for predicting complications, particularly postoperative delirium, in old-old patients | The CGA before gastrointestinal surgery can be a useful tool for predicting the development of postoperative delirium in old-old patients. |
| 10 | Xiang et al. (2017) | China | Prospective cohort | 160 (65 and over) | Colorectal | To investigate the relationship between C-reactive protein (CRP) and postoperative delirium in elderly patients undergoing laparoscopic surgery for colon carcinoma | Preoperative CRP concentrations are predicator for postoperative delirium in patients undergoing laparoscopic surgery for colon carcinoma. |
| 11 | Chen et al. (2017) | Taiwan | Randomized clinical trial | 377 (65 and over) |
Gastric Colorectal Pancreatic |
To examine whether a modified Hospital Elder Life Program (mHELP) reduces incident delirium and length of stay in older adults undergoing abdominal surgery | For older patients undergoing abdominal surgery who received the mHELP, the odds of delirium were reduced by 56% and length of stay was reduced by 2 days. |
1. Definition
2. Prevalence
3. Pathophysiology
Table 3.
| No. | Reference | Preoperative cognitive function definition | Delirium definition | POD prevalence | Prognostic factors & patient outcomes | |
|---|---|---|---|---|---|---|
|
|
||||||
| n | % | |||||
|
|
||||||
| 1 | Olin et al. (2005) | MMSE | CAM; EMR | 26 | 51.0 | Need for intraoperative, postoperative blood transfusion; intraoperative tachycardia; greater intraoperative blood loss; intraoperative crystalloid infusion; postoperative 24h crystalloid infusion; longer operations; longer hospital stay; lower MMSE scores on day 4 after surgery |
| 2 | Morimoto et al. (2009) | HDS; Kana-hiroi-test | DSM-IV; DRS | 5 | 25.0 | Older age; lower preoperative kana-hiroi-test score; lower baseline rSO2; longer recovery time after anesthesia |
| 3 | Koebrugge et al. (2009) | CST | DSM-IV;DOS | 17 | 23.9 | Older than ≥75 years; lower CST scores; ASA score; longer hospital stay; more complications; higher mortality; |
| 4 | Brouquet et al. (2010) | MMSE | CAM | 15 | 18.1 | ASA score 3-4; preoperative hospital stay >2days; ADL score >0; TGUG test >20 seconds; MMSE <26; preoperative calcium serum calcium level<2.2mmol/L; intraoperative blood transfusion; tramadol administration for postoperative analgesia |
| 5 | Patti et al. (2011) | MMSE | CAM; DRS | 18 | 18.0 | Older age; preoperative lower blood albumin levels; intraoperative higher incidence of hypotension; intraoperative elevated infusion volume >5L; intraoperative excessive blood loss; higher mortality; higher complication rates; longer hospitalization stay |
| 6 | Jia et al. (2014) | Cranial MRI scan | DRS-R-98 | 19 | 8.2 | Traditional therapy instead of fast-track surgery; invasive preoperative preparation; opioid drug administration; delayed feeding; enhanced IL-6 level |
| 7 | Tei et al. (2016) | NR | CAM | 44 | 14.1 | Older age; ASA score; performance status; PNI; operative approach; organ/space SSI; cardiac or pulmonary disease |
| 8 | Ito et al. (2016) | NR | DSM-IV | 29 | 19.9 | Older age; medical history of hypertension; CACI score; complication of sepsis; longer hospital stay |
| 9 | Maekawa et al. (2015) | MMSE | CAM | 124 | 24.0 | Barthel Index; vitality index; MMSE; calculated IADL and GDS |
| 10 | Xiang et al. (2017) | MMSE | CAM | 39 | 24.4 | Older age; MCCI; preoperative MMSE score; preoperative or postoperative CRP concentrations; postoperative cardiovascular events |
| 11 | Chen et al. (2017) | MMSE | CAM | 40 | 10.6 | Traditional perioperative care instead of implementing mHELP |
POD=Postoperative delirium; MMSE=Mini mental state examination; HDS=Hasegawa dementia score; CST=Cognitive screening test; MRI=Magnetic resonance imaging; NR=Not reported; CAM=Confusion assessment method; EMR=Electronic medical records; DSM-IV=Diagnostic and statistical manual of mental disorders-IV; DRS=Delirium rating scale; DOS=Delirium observation scale; DRS-R -98=Delirium rating scale-revised-98; ASA=Anesthesiologists physical status classification system; ADL=Activities of daily living; TGUG=Timed get up and go test; PNI=Prognostic nutrition index; Oran/space SSI=Organ space surgical site infection; CACI=Charlson age comorbidity index; IADL=Instrumental activities of daily living; GDS=Geriatric depression score; MCCI=Modified charlson comorbidity index; CRP=C-reactive protein; mHELP=modified hospital elder life program.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print