Factors Influencing Intra-Operative Body Temperature in Laparoscopic Colectomy Surgery under General Anesthesia: An Observational Cohort
Article information
Abstract
Purpose: This study aimed to identify factors influencing intra-operative core body temperature (CBT), and to develop a predictive model for intra-operative CBT in laparoscopic abdominal surgery. Methods: The prospective observational study involved 161 subjects, whose age, weight, and height were collected. The basal pre-operative CBT, pre-operative blood pressure, and heartbeat were measured. CBT was measured 1 hour and 2 hours after pneumoperitoneum. Results: Explanatory factors of intra-operative hypothermia (<36°C) were weight (β=.361, p<.001) and pre-operative CBT (β=.280, p=.001) 1 hour after pneumoperitoneum (Adjusted R2=.198, F=7.56, p<.001). Weight was (β=.423, p<.001) and pre-operative CBT was (β=.206, p=.011) 2 hours after pneumoperitoneum (Adjusted R2=.177, F=5.93, p<.001). The researchers developed a predictive model for intra-operative CBT (°C) by observing intra-operative CBT, body weight, and pre-operative CBT. The predictive model revealed that intra-operative CBT was positively correlated with body weight and pre-operative CBT. Conclusion: Influence of weight on intra-operative hypothermia increased over time from 1 hour to 2 hours after pneumoperitoneum, whereas influence of pre-operative CBT on intraoperative hypothermia decreased over time from 1 hour to 2 hours after pneumoperitoneum. The research recommends pre-warming for laparoscopic surgical patients to guard against intra-operative hypothermia.
INTRODUCTION
Laparoscopic surgery requires carbon dioxide (CO2) gas of 20-21°C for insufflation, which can affect body temperature of surgical patients [1]. In addition, most anesthetics dilate peripheral vessels and increase the flow of heat from the core to peripheral re-distribution [2]. For these reasons, laparoscopic surgery under general anesthesia is likely to be accompanied by intra-operative hypothermia. The incidence of peri-operative hypothermia (<36°C) is as much as 90% in laparoscopic surgery compared to 40-57% in open abdominal surgery [3-5]. In recent years, laparoscopic surgery has been increasingly used in major abdominal and thoracic surgeries.
Hypothermia (<36°C) reduces coagulation enzyme activity, impairs neutrophils, and suppresses activation of lymphocytes and production of cytokines [6-9]. Intra-operative hypothermia might drive surgical wound infection and extend the duration of hospitalization [10,11]. Risk factors of peri-operative hypothermia include older age (>60 years), major surgeries, American Society of Anesthesiologists (ASA) physical status class (≥2), infusion of unwarmed solution or blood transfusion, duration of surgery (>2-3 hours), ambient operating room temperature (<21°C), laparoscopic surgery, and general anesthesia [3,12-14]. Intra-operative warming during laparoscopic surgery helps prevent intra-operative hypothermia [15].
Risk factors of peri-operative hypothermia in laparotomic surgery include pre-operative body temperature, systolic blood pressure, catecholamine level, body weight and body fat [16,17,19]. Even though contributing factors for intra-operative hypothermia in laparotomic surgery have been investigated [3,16,17], factors that influence intra-operative hypothermia for laparoscopic surgery have not been investigated. Therefore, risk factors for intra-operative hypothermia in laparoscopic surgery need to be identified.
The present study was undertaken to investigate the incidence of intra-operative hypothermia and identify the explanatory factors for intra-operative hypothermia in laparoscopic surgery. Pre-operative assessment of risk factors for intra-operative hypothermia in laparoscopic surgery helps to provide individual nursing care to laparoscopic surgical patients and to prevent the patients from peri-operative hypothermia.
METHODS
1. Research design
This study had a prospective descriptive research design. Core body temperature (CBT), heart rate and systolic blood pressure (SBP) were measured before the induction of general anesthesia, and then at 1 hour and 2 hours after pneumoperitoneum in surgical patients who received a laparoscopic colectomy.
2. Subjects and sampling
In this study, inclusion criteria of subjects were age (>20 years), conscious state, and ASA physical status class I or II. We excluded patients with peri-operative use of clonidine (phenothiazine), which can influence thermoregulation, intra-operative blood transfusion, or conditions that can influence metabolic heat production or peripheral vasoconstriction including diabetes mellitus, thyroid disease, and Raynaud’s disease [18,19].
3. Samples and setting
Subjects who received an elective laparoscopic colectomy under general anesthesia were recruited by a convenient sampling at a 1,300-bed G university hospital, Incheon, Korea, from January to June 2012. The subjects in this study included the control group in Park & Yoon’s study [20].
The day before surgery and after the pre-operative interview the principal investigator provided the eligible patients with a printed handout and explained an overview of this study during a face-to-face meeting. A printed handout included the purpose, outcome measurements, duration, and voluntary relinquishment of this study.
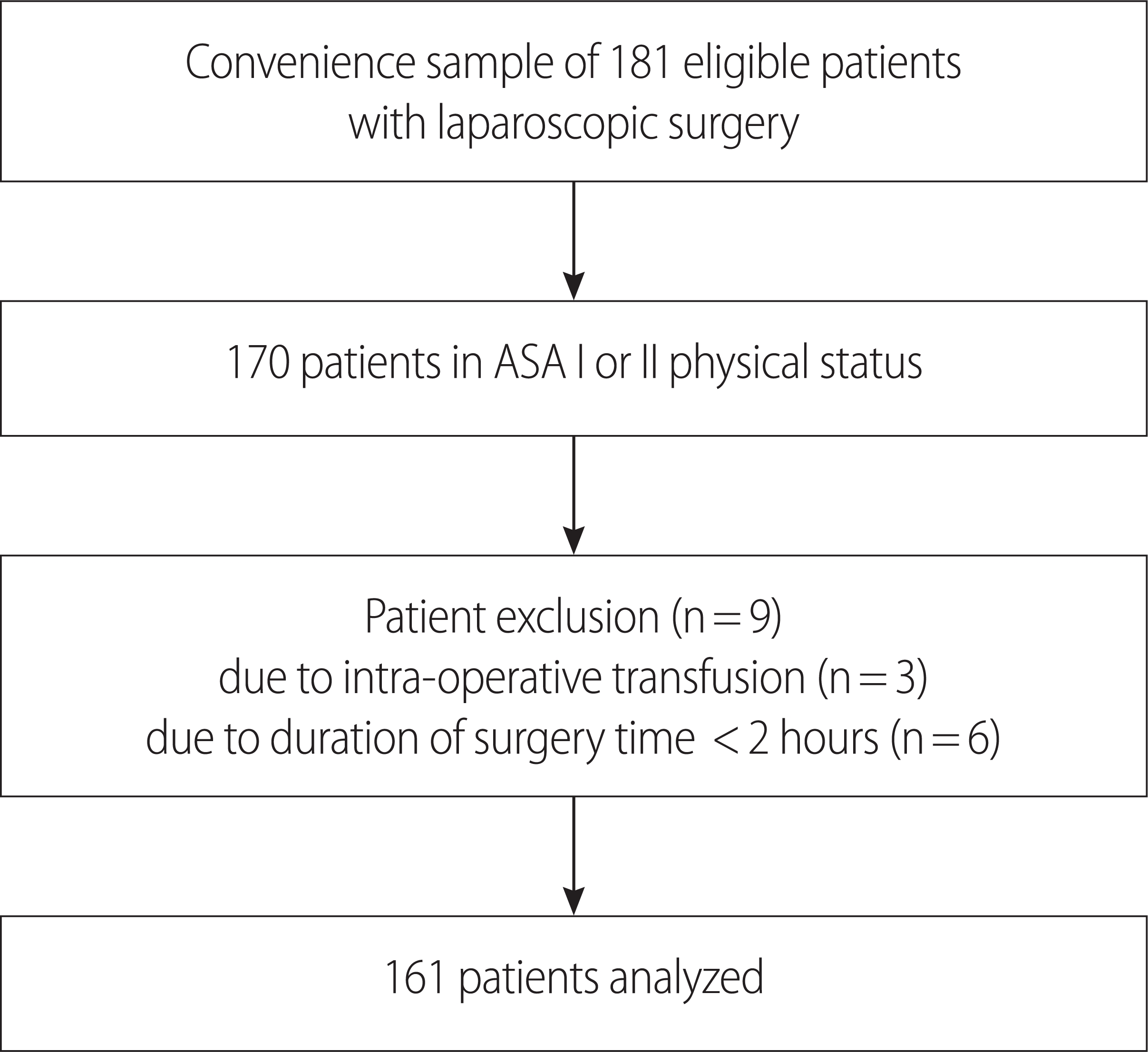
The patients were fully informed about the purpose, design, and duration of this study and could drop-out freely at any time. Written informed consent was waived because it was a non-interventional analysis of our standard practice. Participation was accepted by verbal consent in the light of fulfillment of the inclusion criteria. One-hundred seventy patients provided verbal consent, and nine patients withdrew during the study period, representing a completion rate of 94.7% (Figure. 1).
4. Anesthetic technique
General anesthesia was performed with a standardized technique. All patients were pre-medicated by an intra-muscular injection of glycopyrrolate and/or midazolam. General anesthesia was induced by an intra-venous administration of 1.5 mg/kg propofol, 1 mg/kg rocuronium, and 10 μg alfentanil, and an endotracheal tube was inserted in the trachea. Ventilation was mechanically controlled and fresh gas flow was maintained at 2 L/min by a semi-closed circle system. A heated circuit kit airway heating exchanger (Ace Medical, Seoul, Korea) was set to 40°C. While inhalation anesthesia was maintained with desflurane (6-7%), air (1.5-2 L/min) and O2 (1.5-2 L/min), a blanketrol full-length circulating water mattress set to 38°C (Cincinnati Sub-Zero Products, Cincinnati, OH, USA) was placed under the participant’s back just after induction of general anesthesia, and passive insulation was restricted to surgical draping.
Pneumoperitoneum was established with insufflation of 3 to 4 L of 21°C CO2 into the peritoneal cavity and maintained with an intra-abdominal pressure of 12-15 mmHg. Lactated Ringer’s solution at the ambient temperature of the operating room (22-23°C) was infused at approximately 5-8 mL/kg/h. At the commencement of skin suture, the neuromuscular blockade was reversed with neostigmine (1.0 mg) and atropine (1.0 mg), and the endotracheal tube was removed. Normal saline for irrigation was warmed to 37°C.
5. Power calculation
Effect size based on data of Kim & Yoon [3] and Lee & Yoon [4] was estimated as 0.15. Through G-power 3.1 for multiple regression analysis, a total of 153 subjects were needed with an effect size of 0.15, an alpha of .05, a power of .95 and seven predictors. Seven predictors included age, pre-operative CBT, pre-operative SBP, pre-operative heart rate, weight, total body fat (TBF) and ambient temperature in operating room. Considering a 5% dropout rate, we recruited 170 participants.
6. Data collection
After obtaining approval from the Ethics and Research Committee of G University Hospital (Institutional Review Board No: GIRBA2648), this prospective study was performed on 170 surgical patients. Patients were recruited throughout the general surgery wards after admission and prior to surgery. After surgical patients verbally consented to participate in the study as a research subject, the surgical patients were examined on fulfillment of inclusion criteria for the research subject.
During the pre-operative assessment, demographic data including gender, age, ASA physical status class, disease history, weight, and height (DS-103, Dongsahn Jenix, Seoul, Korea) were collected on the pre-operative visit. Body mass index (BMI, kg/m2) was calculated using the Kauffman index, body surface area (BSA) was calculated by the Dubois index, lean body mass was calculated by the James formula, and total body fat was calculated from lean body mass according to the following formula: [(weight in kg - lean body mass in kg) ÷ weight in kg]×100.
Research assistants were three nurses who had worked more than three years in the anesthetic nursing unit. They measured clinical variables including tympanic membrane CBT, heart rate, and SBP (Table 1). The three assistants were trained to measure tympanic membrane CBT. As soon as the patients were transported to the operating room, basal pre-operative CBT was measured in the right ear using a tympanic membrane thermometer (ThermoScan IRT-4520, Braun, Frankfurter, Germany). The thermometer was calibrated in accordance with manufacturer’s guidelines. As well, the basal pre-operative SBP and heart rate were measured using an electrocardiograph monitor (Dash 4000, GE, New York, NY, USA) in the supine position. CBT was measured again at induction of general anesthesia.
Subsequently, CBT and the ambient temperature in the operating room were measured 1 hour and 2 hours following pneumoperitoneum. In addition, volume of irrigation fluid for the abdominal cavity, and the amount of estimated blood loss were recorded 2 hours after pneumoperitoneum. Finally, the duration of pneumoperitoneum and operation were recorded at the end of the operation.
7. Data analyses
All analyses were performed using IBM SPSS 19.0 (IBM SPSS, Armonk, NY, USA). The chi-square test and Student’s t-test were used to determine differences of the demographic and clinical characteristics between the normothermia (≥ 36°C) and hypothermia (<36°C) groups. The multiple regression through an enter method (p<.05 to enter) was used to determine explanatory factors intra-operative hypothermia in laparoscopic surgery. Variation inflation factors of BMI, BSA, and weight/BSA ratio were more than 10.0, which caused multicollinearity in multiple regression. Therefore, the authors selected weight and TBF as the morphometric variables, and determined a total of seven variables at 2 hours following pneumoperitoneum for an independent variable in the multiple regression.
RESULTS
1. Comparison of general characteristics between normothermia and hypothermia groups
The 161 patients were divided into the normothermia group (≥ 36°C, n=58) and hypothermia group (<36°C, n=103) at 1 hour after pneumoperitoneum, and the normothermia group (n=28) and hypothermia group (n=133) at 2 hours after pneumoperitoneum.
Results of the Student’s t-test and chi-square test for the demographic and clinical physiologic characteristics between the normothermia and hypothermia groups at 1 hour and 2 hours after pneumoperitoneum are presented in Table 2. The Chi-square test for gender, ASA physical status class, and operation type showed no statistically significant differences between the two groups. Student’s t-test for age (p=.003) showed statistically significant differences between either group at 1 hour after pneumoperitoneum. In addition, Student’s t-test for age (p=.004), and ambient temperature (p=.011) showed statistically significant differences between the two groups at 2 hours after pneumoperitoneum.
2. Comparison of physiological characteristics in the normothermia and hypothermia groups
Comparisons of pre-operative CBT, SBP and heart rate between the normothermia and hypothermia groups are presented in Table 3. Student’s t-test revealed statistically significant differences in pre-operative CBT (p<.001), heart rate (p=.013), BMI (p=.003), TBF (p<.001), and weight/BSA ratio (p=.004) at 1 hour after pneumoperitoneum between the two groups. In addition, there were statistically significant differences in pre-operative CBT (p<.001), SBP (p<.001), heart rate (p<.001), weight (p=.003), BMI (p<.001), BSA (p=.049), TBF (p=.024), and weight/BSA ratio (p<.001) at 2 hours after pneumoperitoneum between the two groups.
Mean CBT at 1 hour after pneumoperitoneum was 36.18°C and 35.39°C in the normothermia and hypothermia group, respectively (p<.001). The respective CBT value at 2 hours after pneumoperitoneum was 36.07°C and 35.24°C (p<.001). Of the 161 laparoscopic surgical patients, incidences of core hypothermia (<36°C) at 1 hour and 2 hours after pneumoperitoneum were 64.0% (103 of 161 patients) and 82.6% (133 of 161 patients), respectively.
3. Explanatory factors of intra-operative hypothermia
Table 4 displays the results of the multiple regression analysis to identify the explanatory factors of intra-operative hypothermia (<36°C) at 1 hour and 2 hours after pneumoperitoneum.
There was no autocorrelation problem because the Durbin-Watson statistic (1.59≤d≤1.76) was 1.71 and 1.64 at 1 hour and 2 hours after pneumoperitoneum, respectively. In addition, there was no problem with multicollinearity because tolerance ranged from 0.816 to 0.924 (>.10) and the variance inflation factor ranged from 1.08 to 1.83 (<10) at 1 hour after pneumoperitoneum, tolerance ranged from 0.810 to .944 (>.10), and the variance inflation factor ranged from 1.06 to 1.94 (<10) at 2 hours after pneumoperitoneum. The results of multiple linear regression analysis were considered reliable because all hypotheses of the regression equation, linearity, residual normality, and homoscedasticity were satisfied.
For intra-operative body temperature at 1 hour after pneumoperitoneum, the statistically significant factors were weight (β =.361, p<.001) and pre-operative CBT (β =.280, p=.001). The explanatory power of these two factors was 19.8% (F=7.56, p<.001) (Table 4). For intra-operative body temperature at 2 hours after pneumoperitoneum, the statistically significant factors were weight (β =.423, p<.001) and pre-operative CBT (β =.206, p=.010). The explanatory power of these two factors was 17.7% (F=5.93, p<.001) (Table 4).
We developed a model to predict intra-operative CBT, Y (°C) based on weight (kg) and CBT (°C), X1; body weight (kg), X2: pre-operative CBT (°C)
Y1 (°C)=20.71+0.018×X1 (kg)+0.393×X2 (°C)
Y2 (°C)=23.03 +0.023×X1 (kg)+0.305×X2 (°C)
(Y1=CBT 1 hour after pneumoperitoneum; Y2=CBT 2 hours after pneumoperitoneum)
Therefore, we can estimate intra-operative CBT of 36.13°C 1 hour after pneumoperitoneum of a laparoscopic surgical patient with weight 60 kg and pre-operative CBT of 36.5°C. In addition, we can estimate intra-operative CBT of 35.08°C 2 hours after pneumoperitoneum of a laparoscopic surgical patient with weight 40 kg and pre-operative CBT 36.5°C.
DISCUSSION
The incidences of hypothermia (<36°C) were 64% and 82.6% at 1 hour and 2 hours after pneumoperitoneum in laparoscopic surgery, respectively. Previous studies reported that the incidence of intra-operative hypothermia in laparoscopic surgery had a wider range of 41% up to 94% [21,22]. This wide range seems attributable to a warming method during laparoscopic operation. For example, the incidence of intra-operative hypothermia was 94% in laparoscopic surgical patients who were just covered with blanket mattress [21]. However, the incidence of intra-operative hypothermia was 37% in laparoscopic surgical patients who were actively warmed with forced air warming and airway humidification [22]. According to previous and present findings, peri-operative warming intervention seems effective in reduction of advent intraoperative hypothermia.
The incidences of hypothermia (<36°C) were 64% and 82.6% at 1 hour and 2 hours after pneumoperitoneum in laparoscopic surgery. That is far higher than the rate of 34.7% and 46.3% in laparotomic surgery at 1 hour and 2 hours after induction, respectively [3]. As the present and previous studies were conducted at the same hospital, the research environments were almost similar in several aspects including the use of circulating water mattress set to 38°C, surgical draping type, inhalation anesthesia protocol, and the ambient temperature of the operating room. However, the intra-operative CBT in the present study was 35.39°C and 35.24°C at 1 hour and 2 hours after pneumoperitoneum, respectively, which was lower by 0.21°C and 0.29°C compared with 35.6°C and 35.49°C in the prior study [3]. The National Institute for Health and Care Excellence stipulates a difference of more than 0.2°C is clinically important in CBT less than 36°C [18]. This supports that idea that 21°C carbon dioxide gas during pneumoperitoneum decreases the intra-operative CBT [1]. Throughout abdominal laparoscopic surgery, providing continuously 180-200 L carbon dioxide gas of 21°C [23,24], which is lower than 36.6°C body temperature, seems to have the potential for heat loss and possibly to aggravate intra-operative hypothermia.
Our findings show that the weight and the pre-operative CBT mainly affected the intra-operative CBT during the 2 hours period following pneumoperitoneum. Through this predictive model for intra-operative CBT, CBT of a laparoscopic surgical patient with pre-operative CBT of 36.0°C and 50 kg weight is estimated as 35.75°C and 35.16°C at 1 hour and 2 hours after pneumoperitoneum, respectively. However, when pre-operative body temperature of surgical patient is raised to 37°C through pre-warming, the CBT of surgical patient with 50 kg weight can be estimated as 36.15°C and 35.47°C 1 hour and 2 hours after pneumoperitoneum, respectively. Pre-operative body temperature of 37°C helps raise intra-operative CBT of 0.4°C and 0.31°C at 1 hour and 2 hours after induction compared to pre-operative CBT of 36°C, respectively. Therefore, intra-operative CBT of laparoscopic surgical patient with 40 kg weight and pre-operative CBT of 36°C can fall into severe hypothermia as much as 34.7°C at 2 hours after pneumoperitoneum. Authors are able to assume that pre-warming helps to prevent intra-operative hypothermia in laparoscopic patients through this predictive model for intra-operative CBT.
Some previous studies support our findings that higher pre-operative CBT helps to prevent intra-operative hypothermia and lower body weight brings out intra-operative hypothermia in laparoscopic and laparotomic patients [3,4,13]. Therefore, we suggest warming during transportation or active pre-warming awaiting the operation to increase pre-operative body temperature and prevent intra-operative hypothermia in laparoscopic as well as laparotomic patients [25-27].
Our findings indicate that age, pre-operative SBP, and heart rate did not influence intra-operative hypothermia during the 2 hours after pneumoperitoneum. It has been reported that age, pre-operative SBP, and heart rate influence intraoperative hypothermia in laparotomic surgery [3,4]. Advancing age decreases the responsiveness of the sympathetic nerves to hypothermia [28] and leads to incomplete thermoregulatory vasoconstriction and decreased metabolic heat production [29]. However, presently age did not influence intra-operative hypothermia during the 2 hours after pneumoperitoneum. The influence of age on intra-operative hypothermia in laparoscopic patients seemed negligible compared to laparotomic patients. Those findings imply that continuous provision of carbon dioxide (21°C) during laparoscopic surgery is more influential on intra-operative hypothermia to marginalize the influence of age, pre-operative SBP or heart rate.
The limitation of this study was the administration of glycopyrrolate and/or midazolam as the sedative which can alter threshold temperature. However, this may not be controversial because effects of glycopyrrolate and midazolam on CBT are small compared to the profound impairment induced by general anesthesia [8,30]. Secondly, even though esophageal temperature can be accurate and precise in almost all conditions [6], we measured tympanic membrane CBT because esophageal temperature could not be measured before induction of anesthesia. Even though tympanic membrane temperature can vary according to the measurer, the research assistants were instructed on how to measure the tympanic membrane temperature precisely. Finally, we did not measure the amount of saline used during surgery which could affect intra-operative CBT [26,27]. However, this may not be controversial because the amount of saline used during surgery is small compared to irrigation saline used by the end of operation. Further studies concerning the effect of warm irrigation fluid on post-operative hypothermia are needed.
CONCLUSION
Influential factors on intra-operative hypothermia during 2 hours after pneumoperitoneum seem to include weight and pre-operative CBT. The influence of weight over the intraoperative CBT increased from 1 hour to 2 hours. Therefore, we recommend active pre-warming and intra-operative warming for laparoscopic patients<50 kg weight and duration of surgery>2 hours. Nursing interventions for intra-operative hypothermia can play an important role to decrease adverse outcomes against peri-operative hypothermia.