Inhalation of Clary Sage Oil before Chemotherapy Alleviates Anxiety and Stress without Changing Blood Pressure: A Randomized Controlled Trial
Article information
Abstract
Purpose: The purpose of this study was to evaluate the effects of inhaled clary sage (Salvia sclarea L.) oil or linalyl acetate on patients’ anxiety and stress levels before undergoing chemotherapy. Methods: Forty-five eligible participants were randomly assigned to inhale clary sage oil, or linalyl acetate, each at concentrations of 5% vol/vol in almond oil or pure almond oil (control). State-trait anxiety inventory (STAI), Stress rating scale, anxiety-visual analog scale (Anxiety-VAS), stress-visual analog scale (Stress-VAS), blood pressure, and heart rate were measured before and after the inhalation prior to undergoing chemotherapy. Results: Anxiety-VAS and Stress-VAS were significantly lower after than before inhalation of clary sage oil (p<.01 and p<.05, respectively) and linalyl acetate (p<.05 and p<.05, respectively), despite having no significant difference in the three groups compared with control group. Systolic (p<.05) and diastolic (p<.01) blood pressure before undergoing chemotherapy were significantly lower after than before inhalation of linalyl acetate, while there was no significant difference in after than before inhalation of clary sage oil, despite both reducing levels of anxiety and stress. Conclusion: These findings suggest that linalyl acetate inhalation may be inappropriate in lowering anxiety and stress in patients undergoing chemotherapy, despite its anxiolytic and antistress effects, while clary sage oil inhalation may be useful in reducing anxiety and stress in patients undergoing chemotherapy, which has a risk of hypotensive side effects.
INTRODUCTION
Cancer patients experience psychological distress caused by factors such as pain, altered role in the family, fear of dying, and concerns regarding the side effects of chemotherapy [1]. A large-scale cross-sectional study found that 20% of cancer patients experience psychological distress and that the risk of psychological distress was 1.6-fold higher in cancer patients than in healthy subjects [2]. Similarly, a nationwide cohort study in Korea found that approximately 30% of cancer patients have at least one mental disorder, with half of the latter also experiencing anxiety and somatoform disorders [3]. Anxiety disorders were found to be the most common types of mental disorder occurring after a cancer diagnosis, constituting about 40% of mental disorders [4]. In particular, stress response levels have been reported to be higher in the period before than after chemotherapy in patients with testicular cancer [5]. These findings indicate the importance of psychological interventions for cancer patients, including psychological intervention programs that can be integrated into routine chemotherapy [6].
Despite the prevalence of psychological distress in cancer patients, it is often overlooked, thereby reducing patient compliance [7]. Anxiety and stress reactions can have a negative effect on the efficacy of cancer treatments. For example, the pretreatment anxiety of patients undergoing chemotherapy was shown to be a significant predictor of persistent chemotherapy-induced peripheral neuropathy [8], and exposure to emotional distress was significantly associated with chemotherapy-induced nausea and vomiting [9]. Moreover, the stress-induced secretion of hormones and neurotransmitters has been found to enhance the growth, proliferation, invasion, and metastasis of cancer cells [10] and to reduce the efficacy of chemotherapy [11]. Strategies are therefore needed to reduce anxiety and stress levels in patients undergoing chemotherapy.
Clary sage (Salvia sclarea L.) is an aromatic plant, the essential oil of which has various pharmacological activities. Extract of clary sage manifested anti-inflammatory and antioxidant effects in rats with lipopolysaccharide-induced periodontitis [12]. Compared with inhalation of vehicle or control, inhalation of clary sage oil significantly diminished subjective pain perception in patients with periodontitis [13]. Also, clary sage oil showed antidepressant effects in rats [14]. Moreover, this essential oil is relatively safe, with no adverse effects [15]. Clary sage oil has been shown effective in helping people manage their anxiety and stress. On the elevated plus-maze test, mice fed clary sage oil-enriched feed for 3 months showed a significantly higher number of entries into the open arms of the apparatus than mice fed sunflower oil-enriched feed [16]. In addition, clary sage oil was shown to significantly reduce the corticosterone levels of rats exposed to chronic immobilization stress [17]. The major components of clary sage oil are linalyl acetate (63.7%) and linalool (17.7%) [18], suggesting that these compounds, especially linalyl acetate, may be responsible for the antidepressant and anti-stress effects of clary sage oil. Moreover, mouse inhalation of linalyl acetate was shown to reduce hyperactivity induced by an injection of caffeine, suggesting that linalyl acetate has a sedative effect [19]. Compared with almond oil inhalation, linalyl acetate inhalation reduced pain in patients with indwelling urinary catheters who had undergone colorectal cancer surgery [20].
Taken together, these findings suggested that the inhalation of clary sage oil or linalyl acetate would reduce levels of anxiety and stress in patients prior to undergoing chemotherapy. However, there have been no studies investigating the effects of clary sage oil in cancer patients. The effects of components of essential oil with a low concentration may be greater than those of other components with a high concentration. Also, the effects of the main active components of essential oil may be different from whole essential oil. The present study therefore investigated the effects of inhaled clary sage oil or linalyl acetate diluted in almond oil, respectively, on anxiety and stress levels, heart rate, and blood pressure in patients prior to their first dose of chemotherapy. We hypothesized that inhalation of clary sage oil or linalyl acetate prior to chemotherapy would reduce anxiety and stress levels.
METHODS
1. Study Design
This randomized, placebo-controlled clinical trial enrolled patients with advanced cancer scheduled to undergo chemotherapy from March 2016 to March 2017.
2. Participants
The subjects of this study were patients who had been diagnosed with advanced cancer at a university hospital in Korea and had not yet undergone chemotherapy. The study purpose and procedures were explained to the subjects, with all providing voluntary written consent before enrollment. Patients were included if they (a) were able to communicate, (b) were not allergic to the essential oils included in this study, (c) were not receiving drug treatments, hormone therapy, or aromatherapy for mental disorders, (d) were aged >20 years, and (e) had solid cancers of stage III or higher. Patients were excluded if they (a) had any problems with their olfactory function, or (b) had experience with aromatherapy.
Based on a significance level of 0.05 (one-tailed test), an effect size of 0.5, and a power of 0.8 by one-way ANOVA, and using a G-Power 3.1 program, the minimum sample size required to compare differences among the three groups was 14 per group. The effect size was calculated based on the mean and standard deviation of a previous study reporting that inhalation of clary sage oil provided pain relief in patients with periodontitis [13]. Including a dropout rate of 10%, the number of subjects recruited was 45 subjects. Using a simple random assignment method, 15 subjects were randomly assigned to each group. Of the 45 subjects, 36, or 12 in each group, completed the study.
3. Variable measurement
1) State anxiety
Levels of anxiety were measured using the State Trait Anxiety Inventory (STAI), a self-reported instrument used to measure levels of state and trait anxiety [21] translated into Korean [22]. State anxiety indicates the intensity of an individual’s anxiety response at the time of measurement. Therefore, we used the 20 items of state anxiety to measure patients’ anxiety levels before undergoing chemotherapy. The questionnaire was composed of 10 positive and 10 negative questions for a total of 20 questions. Each question was scored on a 4-point Likert scale, with 1 indicating ‘strongly disagree,’ 2 indicating ‘somewhat agree,’ 3 indicating ‘agree,’ and 4 indicating ‘strongly agree.’ Scores ranged from a minimum of 20 to a maximum of 80 points, with higher scores indicating a higher level of state anxiety. The reliability of the developed instrument had a Cronbach’s α=.92, and the reliability of this study had a Cronbach’s α=.61.
2) Stress rating scale
Levels of stress were measured using a self-administered questionnaire [23], translated into Korean [24]. The questionnaire was composed of 34 questions, with each question scored on a 5-point Likert scale, with 1 indicating ‘do not feel it at all,’ 2 indicating ‘do not feel it much,’ 3 indicating ‘somewhat feel it,’ 4 indicating ‘feel it strongly’, and 5 indicating ‘feel it extremely strongly’; if a question was considered inapplicable, subjects marked it ‘not applicable’, an answer scored as 1 point. Scores ranged from a minimum of 34 to a maximum of 170 points, with higher scores indicating higher level of stress. The reliability of the developed instrument had a Cronbach’s α=.92, and the reliability of the study had a Cronbach’s α=.887 [24].
3) Anxiety-VAS and stress-VAS
Levels of anxiety and stress before and after inhalation were measured on a visual analog scale (VAS), in which subjects indicated their levels of anxiety and stress on 10 cm horizontal lines, with ‘0’ indicating ‘no anxiety or stress’ and ‘10’ indicating ‘extreme anxiety or stress’.
4) Blood pressure and heart rate
In this study, blood pressure and heart rate served as physiological indices of autonomic nervous system activities. Prior to undergoing inhalation and chemotherapy, subjects rested for five minutes in a seated position within a designated space. The blood pressure of the right brachial artery was measured with an electronic blood pressure monitor (ES-H55, Terumo, Tokyo, Japan), and heart rate was calculated by measuring the pulse of their radial artery for one minute. Blood pressure and heart rate were each measured once before and once after inhalation.
4. Intervention
Prior to conducting this study, preliminary investigations were performed to determine the optimal concentrations of clary sage oil and linalyl acetate and the method of inhalation. Five healthy adults who consented to participate were asked to inhale preparations of 1%, 5%, and 10% (v/v) clary sage oil or linalyl acetate in almond oil. These concentrations were found to be non-irritating and to last until the end of treatment. These findings were considered in determining the final concentrations of clary sage and linalyl acetate used in this study. The results of these preliminary experiments were not included in this study.
Clary sage oil and almond oil were obtained from Aromarant Co. Ltd. (batch no. 180622; Rottingen, Germany). Clary sage oil was added to almond oil at a concentration of 5% (v/v). Before inhalation, the suitability of all study subjects was evaluated based on subject selection and exclusion criteria. Each subject participated in a single session of inhalation 20 minutes prior to the start of chemotherapy. None of the participants had breathing problems during these sessions. In the experimental groups, a 1 mL aliquot of 5% clary sage oil or 5% linalyl acetate diluted in almond oil was applied to a piece of gauze, while in the control group, pure almond oil was applied to a piece of gauze. While in a seated position, the piece of gauze was placed under the nose of each subject. Subjects inhaled the scent from the piece of gauze for 20 minutes while breathing naturally. All experiments were performed by a single trained researcher to ensure consistency of assessment; this researcher had been educated about and familiarized with the questionnaire and precautions prior to the study.
Participants were randomly allocated into experimental and placebo groups by block randomization (1:1:1 ratio with a block size of three). The allocation sequence was generated by a randomization function using Microsoft Excel. Clary sage oil and linalyl acetate were prepared by an individual with many years of experience in the manufacture of aromatherapy, not by the investigator who performed the intervention. Clary sage oil, almond oil and linalyl acetate were packaged in identical bottles. To prevent contamination among the participants, they were not informed about the types of substances. Also, all experiments were carried out separately in a separate room to minimize stimuli. The compounder was the only person who knew the assignment of each participant, based on the number on the bottle. Statistical analysts and investigators were blinded to subject assignment.
5. Data Collection
All participating subjects were administered questionnaires assessing general characteristics, anxiety and stress. Subsequently, the levels of anxiety and stress of each subject were measured on a VAS, after which the subject’s blood pressure and heart rate were measured while he or she was in a seated position. Levels of anxiety and stress measured by the VAS, as well as blood pressure and heart rate, were determined before and after inhalation.
6. Statistical Analysis
All statistical analyses were performed using SAS version 9.4. Categorical variables in the three groups were compared by the Chi-square test or Fisher’s exact test. The normality of the continuous variables was determined using the Shapiro-Wilk test, with normally distributed variables compared by analysis of variance (ANOVA) and non-normally distributed variables compared by the Kruskal-Wallis test. Differences among dependent variables were analyzed by the paired t-test, the Kruskal-Wallis test, or ANOVA. A p<.05 indicates statistical significance. In this study, the differences in anxiety and stress levels were numerical mean score differences, not clinically significant differences.
7. Ethical considerations
This study was approved by Clinical Research Ethics Committee of Korea University Anam Hospital (No. ED15338) in Seoul. The study protocol was registered with the Clinical Research Information Service (No. KCT0005485). All study procedures were conducted in accordance with the Declaration of Helsinki (The World Medical Association, 2017), and all participants provided written informed consent.
RESULTS
1. Subject general and disease-related characteristics
Of the 45 subjects recruited, nine dropped out during the study; thus, 36 subjects (12 per group) were included in the final analysis (Figure 1). Their mean age was 53.86 years, their mean body mass index (BMI) was 22.83 kg/m2, and approximately 75% were male, with 50% of patients having stage 3 to 4 gastrointestinal cancers, including gastric, pancreaticobiliary, colorectal, and rectal cancers. There were no significant differences among the three groups in gender distribution, age, BMI, cancer type, and stage of cancer. Levels of state anxiety and stress, as well as anxiety-VAS, stress-VAS, and heart rate, prior to inhalation did not differ significantly among the three groups. In contrast, systolic (p<.05) and diastolic (p<.01) blood pressure of the linalyl acetate inhalation group differed significantly from those of the other two groups (Table 1).
2. Effects of clary sage and linalyl acetate inhalation on anxiety-VAS, stress-VAS, and heart rate
Examination of the changes in anxiety levels from before to after inhalation showed decreases of 0.20±0.73 points in the almond oil group, 1.12±1.22 points in the clary sage oil group, and 0.70±0.78 points in the linalyl acetate group; however, there were no significant differences among the three groups (F=3.87, p=.144). In contrast, changes in anxiety levels from before to after inhalation were statistically significant in the clary sage oil (t=3.18, p<.01) and linalyl acetate (t=3.10, p<.05) groups (Figure 2A).
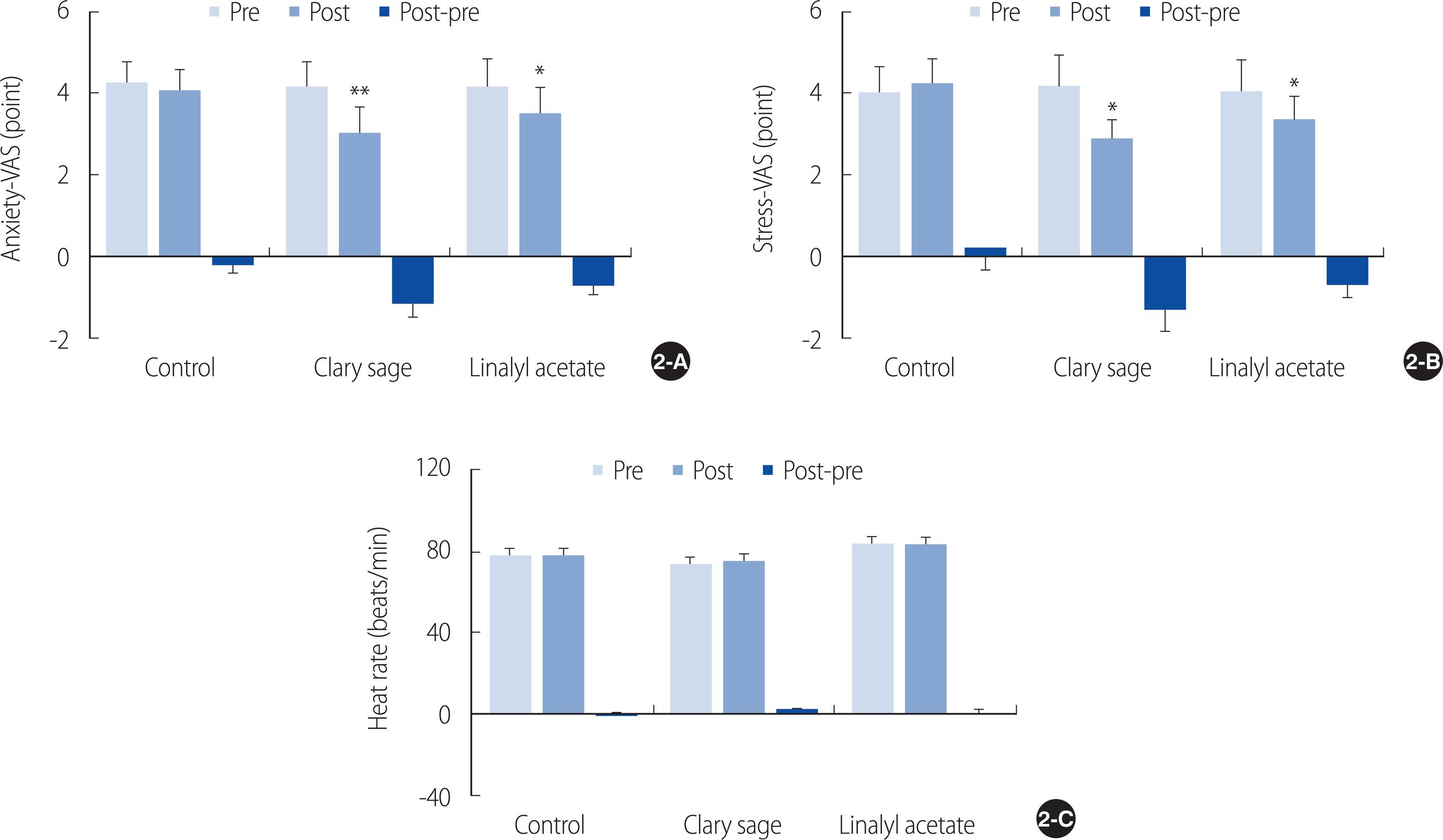
Effect of inhaled clary sage and linalyl acetate on (A) anxiety, (B) stress, and (C) heart rate. Results are expressed as mean±SEM. *p<.05, **p<.01 vs. Pre. VAS=Visual analogue scale.
Stress levels from before to after inhalation increased 0.20±1.83 points in the almond oil group, while decreasing 1.29±1.87 points in the clary sage oil group and 0.70±1.04 points in the linalyl acetate group, but the differences among the three groups were not statistically significant (F=0.83, p=.093). In contrast, changes in stress levels from before to after inhalation were statistically significant in the clary sage oil (t=2.39, p<.05) and linalyl acetate (t=2.33, p<.05) groups (Figure 2B).
Comparisons of heart rates from before to after inhalation showed a 0.33±6.81 beat per minute decrease in the almond oil group, a 1.42±2.94 beat per minute increase in the clary sage oil group, and no change in the linalyl acetate group, but the differences among the three groups were not statistically significant (F=2.19, p=.753). In addition, none of the changes in the individual groups was statistically significant (Figure 2C).
3. Effects of clary sage and linalyl acetate inhalation on systolic and diastolic blood pressure
Comparisons of systolic blood pressure before and after inhalation showed decreases of 5.83±10.60 mmHg in the almond oil group, 0.75±7.74 mmHg in the clary sage oil group, and 7.50±10.35 mmHg in the linalyl acetate group, with no significant differences among the three groups (p=.380). The decrease in systolic blood pressure from before to after inhalation, however, was significant only in the linalyl acetate group (t=2.51, p<.05) (Figure 3A).
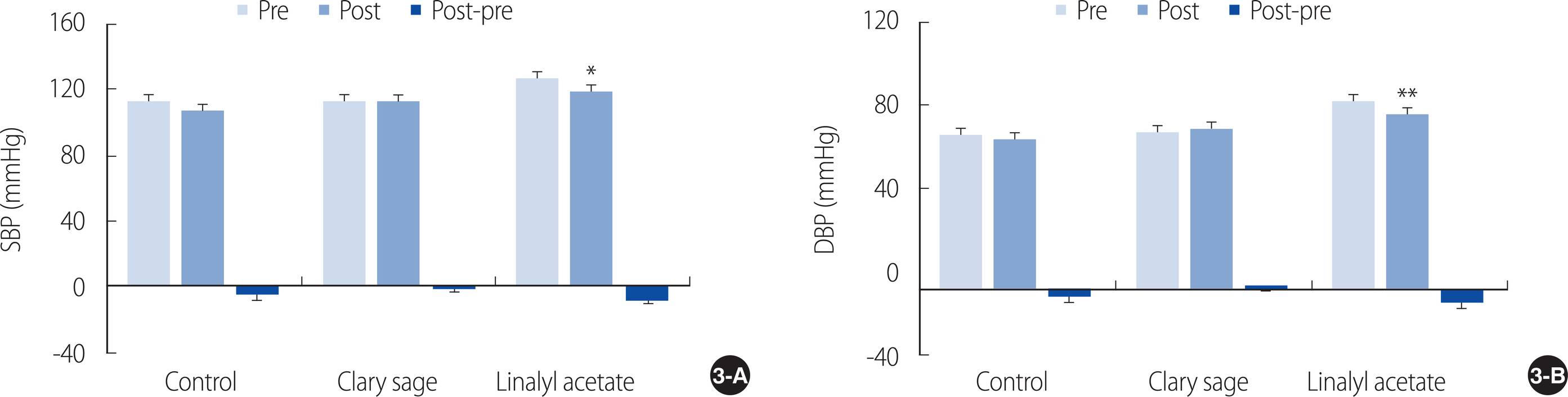
Effect of inhaled clary sage and linalyl acetate on (A) systolic blood pressure and (B) diastolic blood pressure. Results are expressed as mean±SEM. *p<.05, **p<.01 vs. Pre. DBP=Diastolic blood pressure; SBP=Systolic blood pressure.
Similarly, comparisons of diastolic blood pressure before and after inhalation showed decreases of 2.33±8.14 mmHg in the almond oil group and 5.92±6.26 mmHg in the linalyl acetate group, but an increase of 1.67±7.71 mmHg in the clary sage oil group, but these differences were not statistically significant (p=.174). The decrease in diastolic blood pressure from before to after inhalation, however, was significant in the linalyl acetate group (t=3.27, p<.01) (Figure 3B).
DISCUSSION
In addition to cancer itself, chemotherapy can cause psychological distress to patients [1]. Anxiety disorders are the most common mental health disorders in cancer patients, with an incidence ranging from 40% to 65% [4, 25]. Anxiety can cause fatigue, pain, and sleep disorders, and even reduce motivation and coping abilities, thus having a negative effect on patient compliance with treatment plans [26]. Moreover, anxiety is associated with chronic stress, and chronically stressful situations precipitate the occurrence of anxiety disorders [27]. Fatigue is a common symptom experienced by cancer patients undergoing chemotherapy [28], and some of these patients experience cognitive impairment such as memory problems [29].
Various methods have been used to alleviate the levels of anxiety experienced by cancer patients undergoing chemotherapy. For example, pre-chemotherapy education provided by nurses for 30 minutes has been shown effective in reducing anxiety levels [30]. Virtual reality and music therapy were found to significantly reduce levels of anxiety in patients undergoing chemotherapy, although virtual reality programs have been reported to result in symptoms that accompany cyber motion sickness, such as fatigue, dizziness, and headaches [31]. These findings indicate the need for safer methods of intervention that are both easier to implement and are without side effects.
This study evaluated the effects of clary sage oil and linalyl acetate inhalation on levels of anxiety and stress, as well as heart rate and blood pressure, in patients prior to starting chemotherapy. The changes in these parameters from before to after inhalation did not differ significantly in the three groups of patients. However, levels of anxiety and stress were significantly lower after than before inhalation of clary sage oil and linalyl acetate, but not almond oil. These findings are consistent with those showing that inhalation of clary sage oil had a calming and relaxing effect on people, reducing their feelings of anxiety, tension, and agitation [32], as well as reducing the levels of stress experienced by patients with periodontitis [13]. Similarly, levels of anxiety were significantly lower in rats fed clary sage oil-enriched feed than sunflower oil-enriched feed [16].
Linalyl acetate, the main component of clary sage oil [18], appears to be responsible for the reduction of anxiety and stress levels in cancer patients. Linalyl acetate was shown to have an analgesic effect, inhibiting compound nerve action potential [33] and reducing the degree of pain experienced by patients with indwelling urinary catheters who had undergone colorectal cancer surgery [20]. Moreover, linalyl acetate has been reported to have a direct sedative effect [19]. In addition to being the primary component of clary sage oil, linalyl acetate is the main component of lavender [18] and bergamot [34] essential oils, both of which have been shown to reduce levels of anxiety. For example, a meta-analysis found that inhalation of lavender essential oil significantly reduced levels of anxiety in patients [35]. Moreover, inhalation of bergamot essential oil was found to reduce the levels of anxiety in healthy adult women, as well as reducing cortisol concentrations in their saliva [36].
Systemic chemotherapy can cause a variety of adverse effects on the cardiovascular system of patients. Hypotension results from hypersensitivity reactions to drugs such as paclitaxel, etoposide, thalidomide, and interleukin-2 [37]. Although linalyl acetate inhalation significantly reduced blood pressure levels, inhalation of clary sage oil did not, despite both reducing levels of anxiety and stress. Linalyl acetate was found to directly relax vascular smooth muscles by activating the nitric oxide-cyclic guanosine monophosphate pathway and by inhibiting the phosphorylation of myosin light chains [38]. In contrast, inhalation of clary sage oil by pregnant women for 30 minutes increased oxytocin concentrations in their saliva without adverse reactions such as uterine contractions [39]. These findings suggest that the reduction of blood pressure induced by linalyl acetate was inhibited by the antidiuretic effect of oxytocin [40]. This study has several limitations. The sample size was small, and the data were collected only from a single inner-city hospital and patients with solid cancers. Therefore, our findings may not be generalized to other settings. Further studies are needed to include large populations in rural areas or patients with nonsolid cancers.
CONCLUSION
To our knowledge, this study is the first to show that inhalation of 5% clary sage and 5% linalyl acetate effectively reduced patients’ levels of anxiety and stress before the start of chemotherapy. Because chemotherapy can induce hypotension, the inhalation of clary sage oil may be more beneficial, as it effective reduces levels of anxiety and stress without reducing blood pressure. Finally, inhalation of clary sage oil is expected to be actively used as a nursing intervention to reduce levels of anxiety and stress in patients prior to chemotherapy.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHORSHIP
KMS, SYK and SGH contributed to the conception and design of this study; KMS collected data; KMS, SYK, and SGH performed the statistical analysis and interpretation; KMS, SYK and SGH drafted the manuscript; SGH critically revised the manuscript; SGH supervised the whole study process. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF-2021R1A2C2004118) and the Institute of Nursing Research, Korea University Grant. This manuscript is a revision of MK’s master’s thesis from Korea University.